"Executive Summary Viral Vectors-Based Gene Therapy for Non-Human Primates Market :
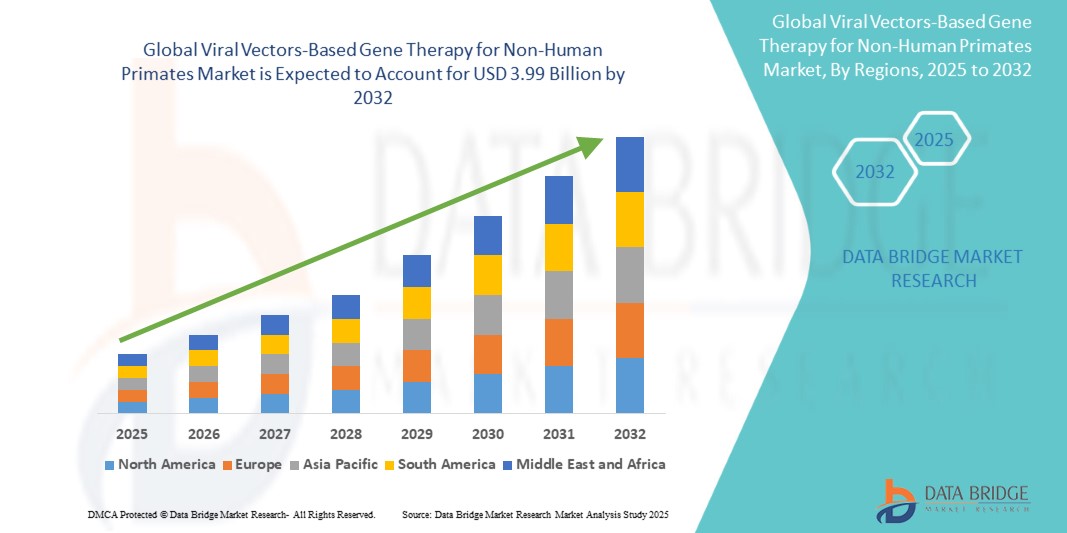
Global viral vectors-based gene therapy for non-human primates market size was valued at USD 1.26 billion in 2024 and is projected to reach USD 3.99 billion by 2032, with a CAGR of 15.40% during the forecast period of 2025 to 2032.
This Viral Vectors-Based Gene Therapy for Non-Human Primates Market report puts light on the market strategies that are being adopted by the competitors and leading organizations. Skilled analysts, statisticians, research experts, enthusiastic forecasters, and economists work together meticulously to structure such a great market research report for the businesses seeking a potential growth. This market report is right there to give out such needs of businesses and hence analyses the market from top to bottom by considering plentiful market parameters. By collecting market research data from different corners of the globe with an experienced team of language resources this global Viral Vectors-Based Gene Therapy for Non-Human Primates Market research report is organized.
The Viral Vectors-Based Gene Therapy for Non-Human Primates Market report can be explored in terms of breakdown of data by manufacturers, region, type and application, market status, market share, growth rate, future trends, market drivers, opportunities and challenges, emerging trends, risks and entry barriers, sales channels, and distributors. This market report is an outcome of incessant efforts lead by clued-up forecasters, innovative analysts and bright researchers who indulge in detailed and attentive research on different markets, trends and emerging opportunities in the consecutive direction for the business needs. It also conducts wide-ranging study about different market segments and regions.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Viral Vectors-Based Gene Therapy for Non-Human Primates Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Overview
**Segments**
- By Vector Type: Adeno-Associated Virus Vectors, Retroviral Vectors, Lentiviral Vectors, Adenoviral Vectors, Other Viral Vectors
- By Application: Gene Therapy, Vaccinology, Functional Genomics, Others
- By End-User: Research Institutes, Biopharmaceutical Companies, Contract Research Organizations, Others
The global viral vectors-based gene therapy for non-human primates market is segmented based on vector type, application, and end-user. Adeno-associated virus vectors are widely used in gene therapy applications for their efficient and safe delivery of genetic material. Retroviral vectors have long been employed for gene therapy due to their ability to integrate into the host genome. Lentiviral vectors are increasingly being utilized for their ability to infect both dividing and non-dividing cells. Adenoviral vectors are commonly used for vaccine development as they efficiently induce immune responses. Other viral vectors, such as herpes simplex virus vectors, are being explored for their unique properties.
In terms of applications, gene therapy holds a significant share in the market as it offers potential cures for genetic disorders by correcting or replacing defective genes. Vaccinology is another key application area where viral vectors are used to deliver vaccine antigens and enhance immune responses. Functional genomics utilizing viral vectors enables the study of gene function and regulation. Other applications include the use of viral vectors in cell reprogramming and stem cell research.
Research institutes play a vital role in driving innovation and advancements in viral vectors-based gene therapy for non-human primates. They are at the forefront of conducting preclinical and clinical studies to evaluate the safety and efficacy of gene therapy approaches. Biopharmaceutical companies are actively engaged in developing viral vector-based therapies for a wide range of diseases, including rare genetic disorders and cancer. Contract research organizations offer specialized services for the preclinical and clinical testing of viral vector-based gene therapies, providing expertise in study design, regulatory compliance, and data analysis.
**Market Players**
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Sirion Biotech GmbH
- Oxford Biomedica
- Sarepta Therapeutics, Inc.
- Lonza
- FUJIFILM Diosynth Biotechnologies
These key market players are actively involved in research and development initiatives to expand their viral vectors-based gene therapy portfolios for non-human primates. Collaborations, partnerships, and acquisitions are common strategies adopted to strengthen their market presence and accelerate product development.
https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-marketThe global viral vectors-based gene therapy for non-human primates market is experiencing significant growth due to the increasing focus on developing advanced therapeutic solutions for genetic disorders and infectious diseases. One of the emerging trends in the market is the utilization of novel viral vector technologies to enhance the delivery efficiency and safety of gene therapy interventions. Advancements in genetic engineering techniques have enabled the development of next-generation viral vectors with improved targeting capabilities and reduced immunogenicity, driving the adoption of viral vector-based gene therapies in both research and clinical settings.
Furthermore, there is a growing emphasis on personalized medicine, and viral vectors are playing a crucial role in delivering tailored gene therapies for individual patients. The ability of viral vectors to deliver genetic material to specific target cells with precision is revolutionizing the treatment landscape for a wide range of diseases. This personalized approach not only improves treatment outcomes but also minimizes the risk of off-target effects, positioning viral vectors as a key technology in the era of precision medicine.
Moreover, the market is witnessing increasing collaborations between academic institutions, research organizations, and biopharmaceutical companies to accelerate the development and commercialization of viral vector-based gene therapies. These partnerships facilitate knowledge sharing, access to cutting-edge technologies, and efficient translation of research findings into clinical applications. By leveraging the collective expertise of various stakeholders, the industry can overcome the challenges associated with viral vector-based gene therapy development, such as vector immunogenicity, safety concerns, and manufacturing scalability.
Additionally, regulatory bodies are playing a proactive role in establishing guidelines and standards for the ethical and safe use of viral vectors in gene therapy applications. Robust regulatory frameworks ensure that viral vector-based therapies meet stringent safety and efficacy requirements before reaching the market, instilling confidence among clinicians, patients, and investors. Compliance with regulatory standards is imperative for market players to navigate the complex landscape of viral vector-based gene therapy development and commercialization successfully.
Overall, the global viral vectors-based gene therapy for non-human primates market is poised for continued growth and innovation as key market players invest in research and development initiatives, forge strategic partnerships, and explore novel applications of viral vector technologies. With advancements in vector design, manufacturing processes, and delivery systems, viral vectors are expected to play an increasingly pivotal role in revolutionizing the treatment of genetic disorders, infectious diseases, and various other therapeutic areas in the coming years.The global market for viral vectors-based gene therapy for non-human primates is witnessing significant growth and advancements driven by the increasing focus on developing cutting-edge therapeutic solutions for genetic disorders and infectious diseases. One of the key trends shaping the market is the adoption of novel viral vector technologies to enhance the efficiency and safety of gene therapy interventions. These advancements in genetic engineering techniques have led to the development of next-generation viral vectors with improved targeting capabilities and reduced immunogenicity, thereby fueling their adoption in both research and clinical applications.
Personalized medicine is another key driver of growth in the market, with viral vectors playing a pivotal role in delivering tailored gene therapies for individual patients. The ability of viral vectors to precisely deliver genetic material to specific target cells is transforming the treatment landscape across a wide range of diseases. This personalized approach not only enhances treatment outcomes but also minimizes the risk of off-target effects, positioning viral vectors as a cornerstone technology in the era of precision medicine.
Collaborations between academic institutions, research organizations, and biopharmaceutical companies are on the rise, aiming to accelerate the development and commercialization of viral vector-based gene therapies. These partnerships facilitate knowledge exchange, access to cutting-edge technologies, and the seamless translation of research discoveries into clinical applications. By harnessing the collective expertise of various stakeholders, the industry can address challenges related to viral vector-based gene therapy development, including immunogenicity, safety concerns, and manufacturing scalability.
Regulatory bodies are also proactively establishing guidelines and standards to ensure the ethical and safe use of viral vectors in gene therapy applications. Robust regulatory frameworks play a crucial role in ensuring that viral vector-based therapies meet stringent safety and efficacy criteria before entering the market, fostering confidence among clinicians, patients, and investors. Compliance with regulatory standards is essential for market players to successfully navigate the complex landscape of viral vector-based gene therapy development and commercialization.
Overall, the global viral vectors-based gene therapy market for non-human primates is poised for continued growth and innovation as key industry players invest in research and development endeavors, establish strategic partnerships, and explore new applications of viral vector technologies. With ongoing advancements in vector design, manufacturing processes, and delivery systems, viral vectors are expected to play an increasingly pivotal role in revolutionizing the treatment landscape for genetic disorders, infectious diseases, and various therapeutic areas in the foreseeable future.
The Viral Vectors-Based Gene Therapy for Non-Human Primates Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Report Investment: Know the Pros
- Besides assessing real time developments and triggers, this section of the report also presents notable past highlights that accelerated growth in this Viral Vectors-Based Gene Therapy for Non-Human Primates Market
- A well scouted presentation of all the crucial segments that collectively harness maximum profit building in global Viral Vectors-Based Gene Therapy for Non-Human Primates Market
- A detailed account of crucial Viral Vectors-Based Gene Therapy for Non-Human Primates Market developments, potential investment bays as well as evaluation of successful business decisions that guide profitable business outcome
- A clear depiction of Viral Vectors-Based Gene Therapy for Non-Human Primates Market specific dynamics, competitor analysis as well as gauging competition intensity
Browse More Reports:
Global Herbal Products Market
Global Tetrakis (Hydroxymethyl) Phosphonium Sulfate Market
Global Insurtech Market
Global Video Management Software (VMS) Market
Global Monochrome Cathode Rays Tube (CRT) Market
Global Flame Photometer Market
Global Structural Insulated Panel Market
Global Serum Market
Global GM1 Gangliosidosis Market
Global Oral Drug Delivery Market
Global Plant Based Feed Enzymes Market
Global Color Cosmetics Market
Global Patient Monitoring Systems Market
Global Aerospace Foam Market
Global Angio Suites (Diagnostic Imaging) Market
Global Automated Dispensing Machines Market
Global Baking Enzymes Market
Global Blue Light Protection Ingredient Market
Global Canned Beans Market
Global Choke Inductor Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Tag
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Size, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Share, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Trend, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Analysis, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Report, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Growth, Latest Developments in Viral Vectors-Based Gene Therapy for Non-Human Primates Market, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Industry Analysis, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Key Player, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Demand Analysis"