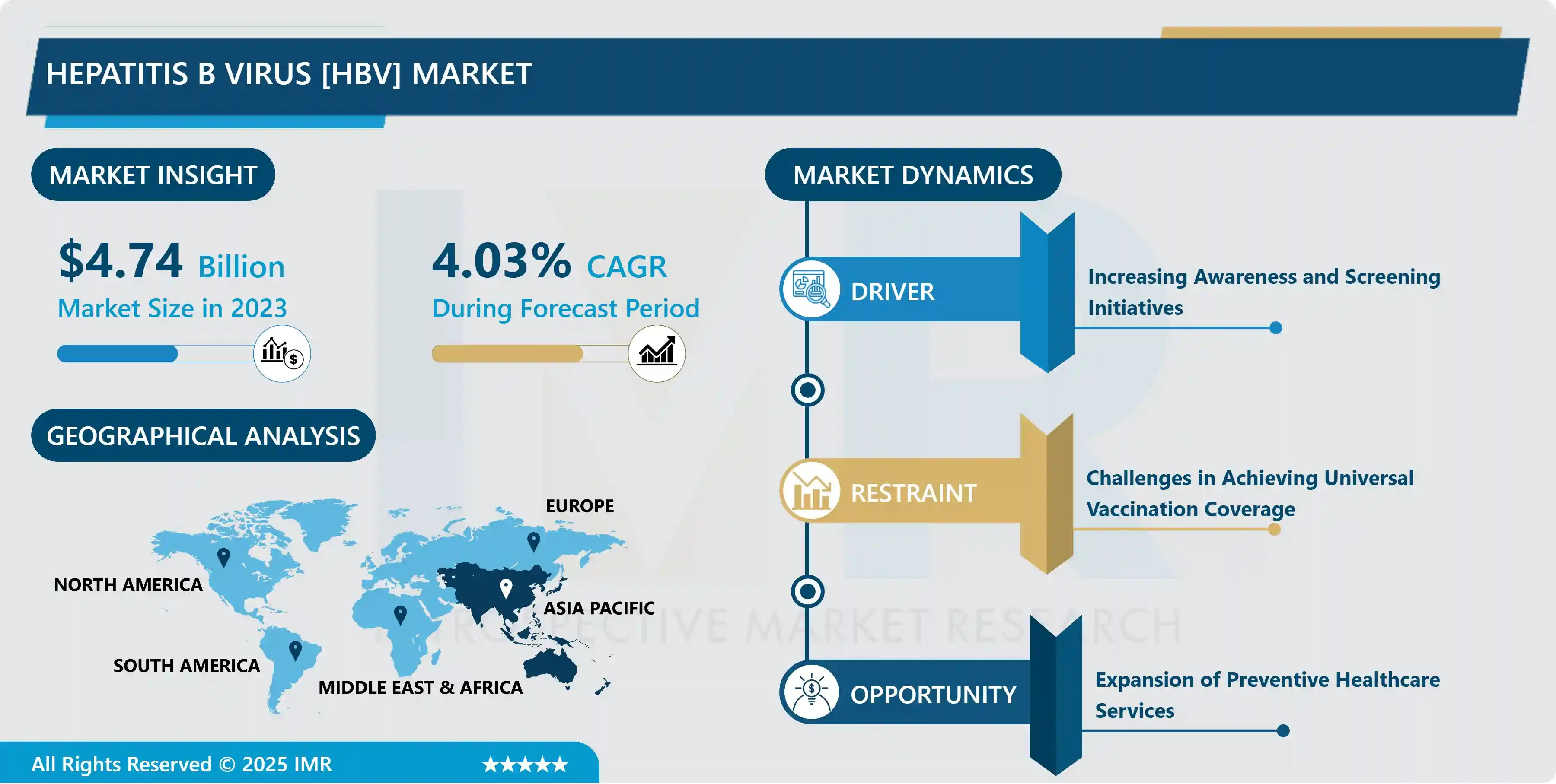
“According to a new report published by Introspective Market Research, Hepatitis B Virus (HBV) Market by Type, Application, and End-User, The Global Hepatitis B Virus (HBV) Market Size Was Valued at USD 4.74 Billion in 2023 and is Projected to Reach USD 6.76 Billion by 2032, Growing at a CAGR of 4.03%.”
Hepatitis B Virus (HBV) is a significant global health concern, causing a viral infection that primarily affects the liver and can lead to both acute and chronic disease. The global HBV market encompasses the ecosystem of diagnostics, vaccines for prevention, and antiviral therapeutics used for the long-term management of chronic infection. The current standard of care for chronic Hepatitis B (CHB) involves nucleoside/nucleotide analogues (NAs) like tenofovir and entecavir, which effectively suppress viral replication and reduce the risk of cirrhosis and liver cancer.
These modern antiviral treatments offer a substantial advantage over older, less potent alternatives by demonstrating high efficacy, good tolerability, and improved long-term patient outcomes, albeit requiring lifelong adherence. The primary uses of these products span from mass vaccination programs for newborns and high-risk groups to sophisticated molecular diagnostics for early detection and disease monitoring in chronic carriers. A strong market imperative is the development of a 'functional cure'where the virus is controlled by the immune system without the need for lifelong drugswhich is fueling immense R&D investment in novel therapeutic classes.
Market Segmentation:
The Hepatitis B Virus (HBV) Market is segmented into Type, Application, and End-User. By Type, the market is categorized into (Vaccines, Therapeutics). By Application, the market is categorized into (Diagnostics, Therapy, Prevention). By End-User, the market is categorized into (Hospitals, Specialty Clinics, Diagnostic Centers, Academic & Research Institutes).
Growth Driver:
The most significant growth driver for the Hepatitis B Virus (HBV) market is the high and increasing global burden of chronic Hepatitis B infection coupled with rising awareness and improved testing accessibility. Despite global vaccination efforts, an estimated 296 million people worldwide are living with chronic HBV infection, creating a sustained and immense demand for long-term antiviral medication. Furthermore, global health organizations and governments are increasingly committed to achieving the World Health Organization’s (WHO) 2030 viral hepatitis elimination goals, which necessitates widespread screening, timely diagnosis, and treatment for eligible individuals. This collective push for proactive disease management is translating directly into higher product uptake and market expansion.
Market Opportunity:
A critical market opportunity lies in the burgeoning field of novel therapeutic development aimed at achieving a functional cure for chronic HBV. Current antiviral treatments suppress the virus but do not eradicate the covalently closed circular DNA (cccDNA), which allows the virus to persist. Therefore, pharmaceutical and biotech companies are heavily investing in next-generation therapeutic candidates, including RNA interference (RNAi) therapies, capsid assembly modulators (CAMs), and various immunotherapies. The successful launch and commercialization of a functional cure, which would command a premium price and transform the current lifelong treatment model, represents the single largest future growth catalyst and market opportunity.
Detailed Segmentation:
Hepatitis B Virus (HBV) Market, Segmentation The Hepatitis B Virus (HBV) Market is segmented on the basis of Type, Application, and End-User.
Type
The Type segment is further classified into Vaccines and Therapeutics. Among these, the Therapeutics sub-segment accounted for the highest market share in 2023. The dominance of the Therapeutics segment is driven by the vast patient pool already diagnosed with chronic HBV infection who require continuous, often lifelong, antiviral treatment. These medications, primarily nucleoside/nucleotide analogues like tenofovir and entecavir, are essential for suppressing viral replication, halting disease progression, and preventing fatal complications such as liver cirrhosis and hepatocellular carcinoma. The high volume and chronic nature of prescription for these drug regimens contribute significantly to the segment’s leading revenue share.
Application
The Application segment is further classified into Diagnostics, Therapy, and Prevention. Among these, the Therapy sub-segment accounted for the highest market share in 2023. The Therapy application segment's leading position is directly linked to the high expenditure on long-term drug regimens for chronic HBV patients. Therapy, which includes the continuous dispensing of antiviral drugs, is an ongoing revenue stream for the market. While diagnostics and prevention (vaccination) are crucial for market entry and population health, the established and chronic need for suppressive medication among millions of infected individuals makes the Therapy segment the largest in terms of market value.
Some of The Leading/Active Market Players Are-
- Gilead Sciences, Inc. (United States)
- GlaxoSmithKline plc (United Kingdom)
- Bristol-Myers Squibb Company (United States)
- Novartis AG (Switzerland)
- Hoffmann-La Roche Ltd (Switzerland)
- Merck & Co., Inc. (United States)
- Dynavax Technologies Corporation (United States)
- VBI Vaccines Inc. (United States)
- Arbutus Biopharma Corporation (United States)
- Assembly Biosciences, Inc. (United States)
- Vir Biotechnology, Inc. (United States)
- Takeda Pharmaceutical Company Limited (Japan)
- Johnson & Johnson (Janssen) (United States)
- Precision Biosciences (United States)
- Aligos Therapeutics, Inc. (United States)
...and other active players.
Key Industry Developments
In February 2024, GSK plc received the Fast Track designation from the U.S. FDA for its investigational antisense oligonucleotide, bepirovirsen, for the treatment of chronic hepatitis B (CHB).
This designation is crucial as it facilitates the development and speeds up the review of drugs intended to treat serious conditions and fill an unmet medical need. Bepirovirsen is a potential functional cure component, representing the industry’s shift away from purely suppressive drugs toward agents that can clear the Hepatitis B surface antigen (HBsAg) and offer a long-term, medication-free remission for patients.
In October 2023, Gilead Sciences, Inc. and Assembly Biosciences signed a major 12-year partnership agreement to advance the research and development of novel anti-viral therapies, including a significant focus on the Hepatitis B Virus (HBV).
This strategic collaboration merges Gilead’s commercial and development capabilities with Assembly Biosciences’ expertise in developing next-generation compounds like capsid assembly modulators (CAMs). The partnership is a clear indicator of the pharmaceutical industry’s commitment to accelerating the discovery of combination therapies required to achieve the elusive functional cure for chronic HBV infection.
Key Findings of the Study
- The Therapeutics segment, driven by lifelong antiviral drug use, holds the dominant market share.
- North America remains the leading region in terms of market value, supported by high healthcare spending and a robust pipeline of novel drugs.
- The primary growth driver is the high global burden of chronic HBV infection and rising screening/treatment awareness.
- A key market trend is the intense focus on developing functional cure agents (like RNAi and CAMs) to eliminate the virus and enable treatment cessation.
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Hepatitis B Virus (HBV) Market . With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global HBV Market industry.
📞 Contact Us Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569