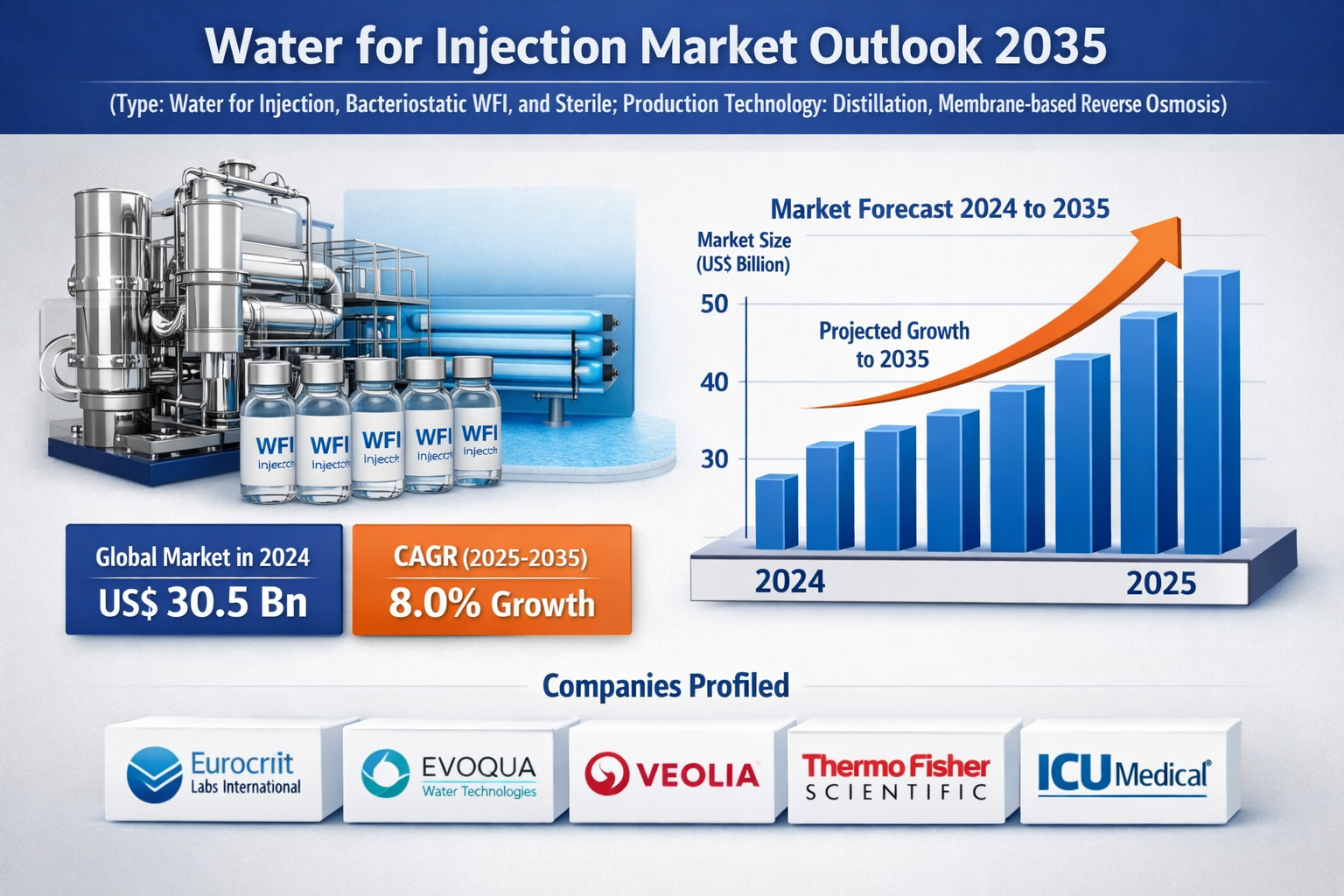
The global water for injection market was valued at USD 30.5 billion in 2024 and is projected to grow at a CAGR of 8.0% from 2025 to 2035, driven by rising pharmaceutical and biotechnology production, increasing demand for sterile injectable drugs, and stringent regulatory standards for drug manufacturing. Market growth is further supported by expanding vaccine production, growth in biologics, and continuous investments in high-purity water generation and distribution systems.
Increase in demand for biopharmaceutical & biosimilar and growing adoption of single-use systems in pharmaceutical manufacturing is anticipated to boost water for injection market growth. Water for injection is extremely pure, sterile water employed in the manufacture of injectable pharmaceuticals, vaccines, and biologics to ensure drug safety and regulatory compliance. Its significance is to preserve the integrity and potency of parenteral pharmaceuticals by avoiding contamination.
Transform Your Strategy: Explore In-Depth Data – Sample Available! https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=84885
The global water for injection (WFI) market is experiencing significant growth, driven by the rising demand for biopharmaceuticals and biosimilars. WFI of high purity is necessary in the manufacturing of biologics like monoclonal antibodies, vaccines, and gene therapies where sterility is a prime factor for ensuring product safety and effectiveness. Growing application of biologics for chronic disease, autoimmune diseases, and cancer treatment is driving demand for WFI.
Market Segmentation
The WFI market is multifaceted, categorized by how it is produced, packaged, and utilized across various healthcare verticals.
| Segment Category | Key Sub-segments |
| By Type | Sterile WFI, Bacteriostatic WFI, Purified WFI, Single/Double Distilled WFI |
| By Sourcing/Production | Distillation (Traditional), Membrane-based (Reverse Osmosis), Hybrid Systems |
| By Application | Drug Formulation (Excipient), Reconstitution, Equipment Cleaning, Culture Media |
| By Industry Vertical | Pharmaceutical Companies, Biotechnology Firms, CMOs/CDMOs, Research Institutes |
| By Packaging | Vials & Ampoules, Bottles, Pre-filled Syringes, Bulk Containers/Drums |
Regional Analysis: A Global Shift
- North America: Remains the dominant region, holding roughly 40% of the market share. This is driven by advanced manufacturing infrastructure and a high concentration of biopharmaceutical giants in the U.S.
- Asia-Pacific: Projected to be the fastest-growing region with a CAGR exceeding 10%. Rapid expansion in China and India, fueled by government initiatives to improve healthcare and a surge in local manufacturing of biosimilars, is repositioning this region as a global WFI hub.
- Europe: A steady leader in innovation, particularly in sustainable and energy-efficient WFI production technologies, maintaining a significant portion of the global share.
Market Drivers and Challenges
Drivers
- Biopharmaceutical Boom: The shift from small molecules to complex biologics and gene therapies requires ultra-pure water that meets the highest safety standards.
- Stringent Regulatory Standards: Compliance with USP, EP, and WHO guidelines is non-negotiable, forcing manufacturers to invest in high-end, validated WFI systems.
- Rise of Single-Use Technologies: The adoption of disposable manufacturing systems reduces cross-contamination and drives the demand for pre-packaged, sterile WFI solutions.
Challenges
- High Energy Consumption: Traditional distillation processes are energy-intensive, posing a challenge to the "green" goals of modern pharmaceutical companies.
- Maintenance & Biofilm Risks: Constant monitoring is required to prevent microbial growth in storage loops, which can lead to expensive batch failures.
- Capital Investment: Setting up compliant WFI generation and distribution systems requires significant upfront capital.
Key Market Trends
- Transition to Membrane-based WFI: Many facilities are moving away from traditional distillation toward "Cold WFI" technologies (Reverse Osmosis + Electrodeionization + Ultrafiltration), which are more energy-efficient.
- AI and Real-time Monitoring: The integration of AI for predictive maintenance and real-time Total Organic Carbon (TOC) monitoring is becoming a standard for "Pharma 4.0" facilities.
- Outsourcing to CDMOs: Small biotech firms are increasingly relying on Contract Development and Manufacturing Organizations (CDMOs), boosting bulk WFI consumption in specialized hubs.
Competitive Landscape
The market is characterized by a mix of established healthcare giants and specialized water technology providers.
- Major Pharmaceutical/Healthcare Players: Baxter International, Pfizer, B. Braun Melsungen AG, Merck KGaA, and Johnson & Johnson.
- Technology & System Leaders: BWT Holding GmbH, Veolia Water Technologies, Evoqua (Aqua-Chem), STERIS, and MECO.
Recent Developments
- In 2024, Veolia Water Technologies has launched the latest generation of Polaris, its leading high-capacity water distillation and steam generation solutions, developed for the pharmaceutical industry. Using a range of standard options, the skid-mounted PoIaris 2.0 Multiple Effect Distiller (MED) and Polaris 2.0 Pure Steam Generator (PSG) systems have been engineered to reliably deliver the required volumes of water for injection (WFI) and pure steam in line with European, Japanese and US Pharmacopoeia standards.
- In 2022, WuXi STA, has announced its first parenteral formulation manufacturing line at the Wuxi city site. In this manufacturing plant fully automatic sterile manufacturing line operates in a full isolation system with an annual capacity of 2 million units.
Future Outlook & Key Study Points
The period between 2025 and 2035 will be defined by Sustainability and Automation. We expect to see WFI systems that are nearly autonomous, using IoT sensors to self-correct water quality deviations before they reach the production line. As global health initiatives prioritize vaccination, the demand for WFI as a diluent will remain a cornerstone of public health infrastructure.
Elevate Your Business Strategy! Purchase the Report for Market-Driven Insights: https://www.transparencymarketresearch.com/checkout.php?rep_id=84885<ype=S
Key Study Points for Investors:
- Monitor the regulatory shift toward membrane-based WFI in the European Pharmacopoeia.
- Watch for M&A activity between water technology firms and digital AI startups.
- Keep an eye on the emerging "at-home" injectable market, which increases the demand for smaller, pre-filled WFI formats.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com